All of the Following Can Be Considered Physical Properties Except:
Chapter 1. Essential Ideas
i.3 Physical and Chemic Properties
Learning Objectives
Past the end of this department, you will be able to:
- Identify backdrop of and changes in matter equally physical or chemical
- Place backdrop of matter as extensive or intensive
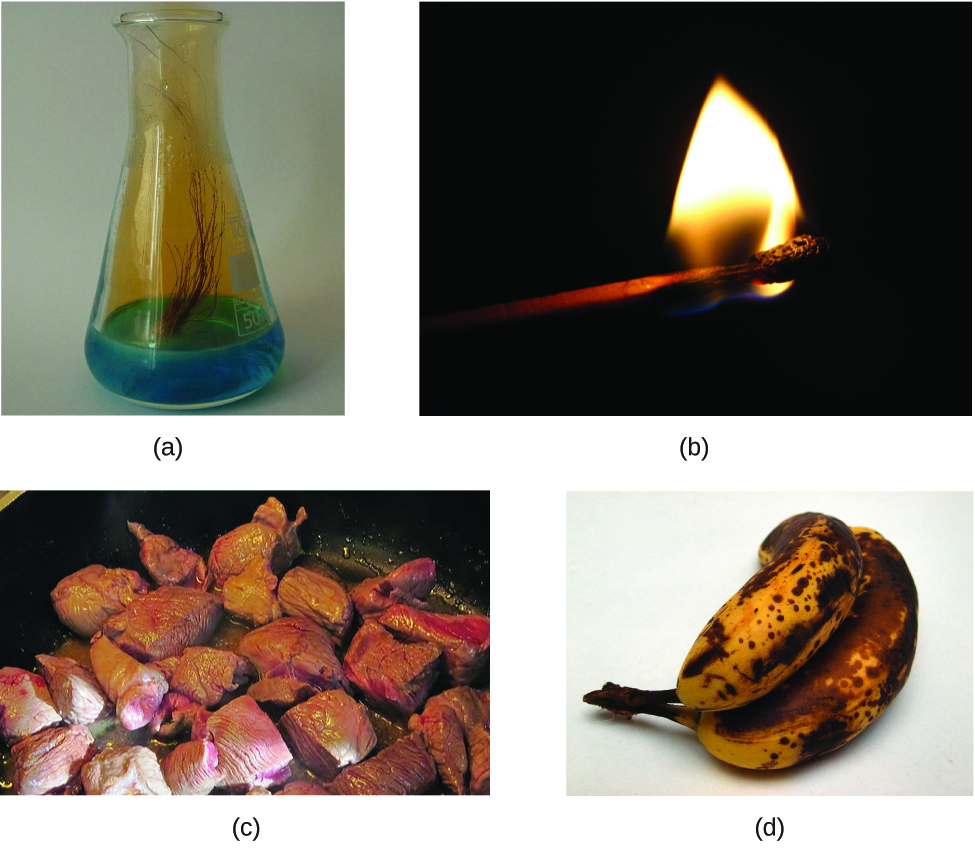
The characteristics that enable united states to distinguish one substance from another are called properties. A concrete property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points, and conductivity. We can observe some physical backdrop, such as density and color, without changing the physical state of the affair observed. Other physical properties, such as the melting temperature of fe or the freezing temperature of water, can merely be observed as thing undergoes a concrete change. A physical change is a change in the state or properties of matter without whatever accompanying modify in its chemical composition (the identities of the substances contained in the affair). Nosotros observe a physical change when wax melts, when saccharide dissolves in java, and when steam condenses into liquid h2o (Effigy 1). Other examples of physical changes include magnetizing and demagnetizing metals (equally is washed with common antitheft security tags) and grinding solids into powders (which can sometimes yield noticeable changes in color). In each of these examples, there is a alter in the concrete state, form, or properties of the substance, but no change in its chemic limerick.

The alter of one type of thing into another type (or the inability to change) is a chemical property. Examples of chemical properties include flammability, toxicity, acidity, reactivity (many types), and heat of combustion. Fe, for example, combines with oxygen in the presence of h2o to class rust; chromium does not oxidize (Figure ii). Nitroglycerin is very dangerous considering information technology explodes hands; neon poses almost no hazard because it is very unreactive.

To identify a chemical property, we look for a chemical modify. A chemical change always produces one or more types of matter that differ from the matter sspresent before the change. The formation of rust is a chemical change because rust is a dissimilar kind of matter than the iron, oxygen, and water present before the rust formed. The explosion of nitroglycerin is a chemical change because the gases produced are very dissimilar kinds of affair from the original substance. Other examples of chemical changes include reactions that are performed in a lab (such as copper reacting with nitric acid), all forms of combustion (burning), and nutrient being cooked, digested, or rotting (Figure 3).
Properties of matter fall into one of two categories. If the property depends on the amount of affair present, it is an extensive property. The mass and volume of a substance are examples of extensive backdrop; for case, a gallon of milk has a larger mass and volume than a cup of milk. The value of an extensive belongings is directly proportional to the corporeality of matter in question. If the property of a sample of matter does non depend on the corporeality of matter nowadays, it is an intensive property. Temperature is an example of an intensive property. If the gallon and cup of milk are each at xx °C (room temperature), when they are combined, the temperature remains at xx °C. Every bit some other example, consider the distinct but related properties of heat and temperature. A drop of hot cooking oil spattered on your arm causes brief, minor discomfort, whereas a pot of hot oil yields severe burns. Both the drop and the pot of oil are at the same temperature (an intensive holding), simply the pot conspicuously contains much more than heat (all-encompassing property).
Chance Diamond
Yous may have seen the symbol shown in Effigy iv on containers of chemicals in a laboratory or workplace. Sometimes chosen a "fire diamond" or "take a chance diamond," this chemical hazard diamond provides valuable information that briefly summarizes the diverse dangers of which to be aware when working with a particular substance.
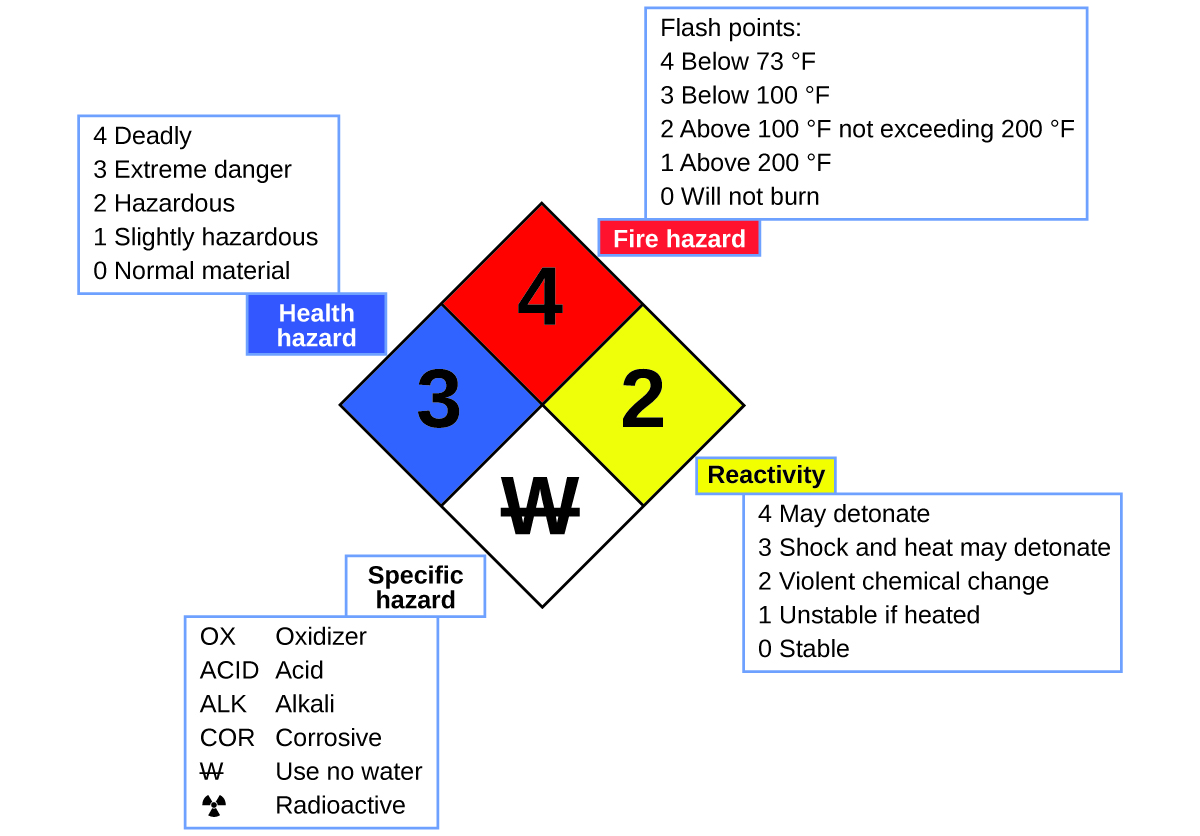
The National Burn down Protection Agency (NFPA) 704 Hazard Identification System was developed by NFPA to provide safety information about sure substances. The arrangement details flammability, reactivity, health, and other hazards. Within the overall diamond symbol, the acme (carmine) diamond specifies the level of burn down hazard (temperature range for wink point). The bluish (left) diamond indicates the level of health hazard. The yellow (right) diamond describes reactivity hazards, such as how readily the substance volition undergo detonation or a violent chemical change. The white (bottom) diamond points out special hazards, such every bit if it is an oxidizer (which allows the substance to fire in the absence of air/oxygen), undergoes an unusual or unsafe reaction with h2o, is corrosive, acidic, alkaline, a biological gamble, radioactive, and so on. Each take chances is rated on a scale from 0 to iv, with 0 being no gamble and four existence extremely chancy.
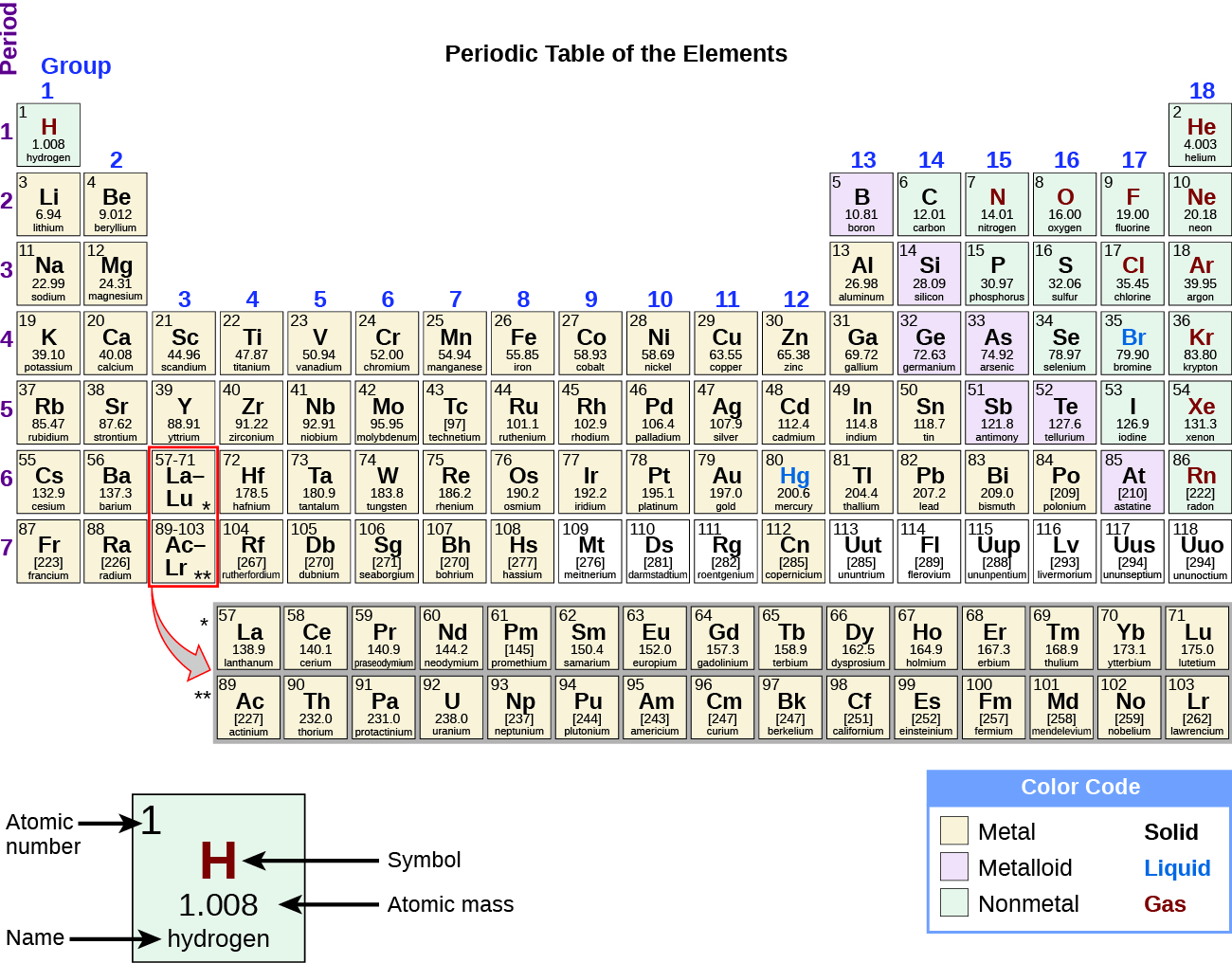
While many elements differ dramatically in their chemical and concrete backdrop, some elements have similar properties. Nosotros can identify sets of elements that exhibit common behaviors. For example, many elements conduct heat and electricity well, whereas others are poor conductors. These properties can be used to sort the elements into three classes: metals (elements that comport well), nonmetals (elements that comport poorly), and metalloids (elements that have properties of both metals and nonmetals).
The periodic table is a tabular array of elements that places elements with similar properties close together (Figure 4). You volition larn more about the periodic table as you lot go along your study of chemistry.
Key Concepts and Summary
All substances take distinct physical and chemical backdrop, and may undergo physical or chemic changes. Concrete backdrop, such equally hardness and boiling signal, and physical changes, such every bit melting or freezing, exercise non involve a change in the composition of matter. Chemical backdrop, such flammability and acerbity, and chemic changes, such as rusting, involve production of matter that differs from that present beforehand.
Measurable properties fall into one of ii categories. All-encompassing backdrop depend on the amount of matter present, for instance, the mass of gold. Intensive properties do not depend on the corporeality of thing present, for example, the density of gilded. Heat is an example of an extensive property, and temperature is an case of an intensive property.
Chemistry Stop of Chapter Exercises
- Classify the half dozen underlined properties in the post-obit paragraph as chemical or physical:
Fluorine is a pale yellow gas that reacts with most substances. The complimentary element melts at −220 °C and boils at −188 °C. Finely divided metals burn in fluorine with a bright flame. Nineteen grams of fluorine will react with ane.0 gram of hydrogen.
- Classify each of the following changes equally physical or chemic:
(a) condensation of steam
(b) burning of gasoline
(c) souring of milk
(d) dissolving of saccharide in h2o
(e) melting of gilt
- Classify each of the post-obit changes as physical or chemical:
(a) coal burning
(b) water ice melting
(c) mixing chocolate syrup with milk
(d) explosion of a firecracker
(east) magnetizing of a screwdriver
- The volume of a sample of oxygen gas inverse from ten mL to eleven mL as the temperature changed. Is this a chemical or physical change?
- A ii.0-liter volume of hydrogen gas combined with 1.0 liter of oxygen gas to produce 2.0 liters of h2o vapor. Does oxygen undergo a chemical or physical change?
- Explicate the departure between extensive properties and intensive properties.
- Identify the following properties as either extensive or intensive.
(a) book
(b) temperature
(c) humidity
(d) heat
(due east) humid point
- The density (d) of a substance is an intensive holding that is defined as the ratio of its mass (m) to its volume (5).
[latex]\text{density}= \frac{\text{mass}}{\text{volume}}[/latex] [latex]\text{d} = \frac{\text{k}}{\text{Five}}[/latex]
Considering that mass and volume are both extensive properties, explain why their ratio, density, is intensive.
Glossary
- chemical change
- change producing a different kind of matter from the original kind of matter
- chemical property
- behavior that is related to the change of one kind of matter into another kind of affair
- extensive property
- property of a substance that depends on the corporeality of the substance
- intensive property
- property of a substance that is contained of the amount of the substance
- physical change
- change in the state or properties of matter that does not involve a alter in its chemical composition
- physical property
- feature of matter that is not associated with any change in its chemical limerick
Solutions
Answers for Chemistry Cease of Chapter Exercises
2. (a) physical; (b) chemical; (c) chemic; (d) physical; (due east) concrete
4. concrete
6. The value of an extensive belongings depends upon the amount of matter being considered, whereas the value of an intensive property is the same regardless of the amount of matter being considered.
8. Being extensive properties, both mass and volume are directly proportional to the amount of substance under report. Dividing 1 all-encompassing property by another will in effect "cancel" this dependence on corporeality, yielding a ratio that is independent of amount (an intensive belongings).
Source: https://opentextbc.ca/chemistry/chapter/physical-and-chemical-properties/
0 Response to "All of the Following Can Be Considered Physical Properties Except:"
Postar um comentário